The future of hydrogen fuel: UNSW researchers develop technique to analyse hydrogen fuel cell stability
Understanding why existing hydrogen fuel cell prototypes degrade can pave the way for more durable cells.╠ř╠ř
Understanding why existing hydrogen fuel cell prototypes degrade can pave the way for more durable cells.╠ř╠ř
Lilly Matson
UNSW Science
0426 656 007
l.matson@unsw.edu.au
Scientists at UNSW Sydney are working on ways to improve the efficiency and cost of hydrogen fuel cells, to increase access to clean fuel.╠ř╠ř
Hydrogen has been positioned as a key player in the race to a decarbonised future, but despite its potential, the road to commercialisation has been a slow one.╠ř╠ř
There are several factors that scientists ÔÇô including , and Mr Shiyang Liu from the School of Chemistry at UNSW ÔÇô are trying to address, to increase the commercial viability of hydrogen fuel cells.╠ř
There are issues with the cost and resources of some of the key elements that make up a hydrogen fuel cell, including platinum, the material commonly used as the catalyst needed to activate the process. Creating alternatives to platinum catalysts is essential.╠ř╠ř
ÔÇťPlatinum is always going to be expensive, because there isn't a lot out there,ÔÇŁ says Prof. Zhao. ÔÇťSo, we need to explore alternatives, whilst also providing a quick and easy way to measure how well these new materials are working in hydrogen fuel cells.ÔÇŁ╠ř
In a new study, published in , Prof. ZhaoÔÇÖs team has developed a novel process to test the durability and stability of platinum alternatives that will supply new insights into cost-friendly options for hydrogen fuel cells.╠ř
Hydrogen fuel cells, which were developed as a green energy source in the 19th century, use chemical reactions to break hydrogen into protons and electrons, producing electricity and water.╠ř╠ř
Read more: ╠ř
ÔÇťEssentially you have two sides ÔÇô an anode and a cathode. You put hydrogen on one side (the anode) and oxygen on the other (the cathode),╠řand catalysts that make the two reactions happen,ÔÇŁ says Dr Meyer. ÔÇťOne reaction is splitting hydrogen into protons and electrons, and then the other side oxidising oxygen. The protons and electrons react with oxygen at the cathode to make water and electricity.ÔÇŁ╠ř
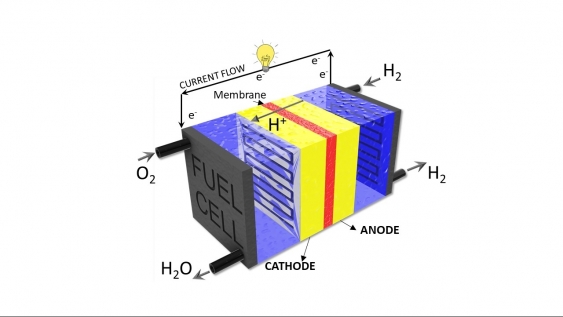
Hydrogen fuel cells use chemical reactions to break hydrogen into protons and electrons to produce electricity and water. Image: Hydrogen fuel cell schematic with the anode and cathodeÔÇő. Supplied.
The key difference between hydrogen fuel cell technology and batteries is that you donÔÇÖt need to charge hydrogen fuel cells up. Instead of a petrol pump, you just have a hydrogen pump and it takes only three minutes to refuel a hydrogen fuel cell car.╠ř
The process is not only considered a clean source of energy, only producing water as a by-product, but it is also sustainable. Hydrogen itself is a very abundant element, and while it doesnÔÇÖt occur naturally, it can be extracted from water.╠ř╠ř
ÔÇťWe are faced with a ÔÇśchicken or the eggÔÇÖ type problem, where we donÔÇÖt have enough hydrogen being processed, or enough places to use the hydrogen once itÔÇÖs been extracted,ÔÇŁ says Prof. Zhao. ÔÇťSo, as we start to produce more hydrogen and more fuel cells, then both will become cheaper.ÔÇŁ╠ř
Another key problem is the cost of the catalyst. The platinum which forms the essential middle layer of a fuel cell costs somewhere between .╠ř╠ř
ÔÇťOne approach is to use platinum alternatives, such as iron, which only costs around ÔÇŁ says Mr Liu, ÔÇťA particular promising material is Iron-nitrogen-carbon, also known as Fe-N-CÔÇŁ╠ř╠ř
However, these new platinum alternatives are not currently widely available because they are not as stable as platinum and break down at a faster rate in hydrogen fuel cells. ÔÇťWhile platinum-based fuel cells can last up to 40,000 hours (about 4 and a half years), the iron-nitrogen-carbon materials can only run up to 300 hours (about 2 weeks), in a best-case-scenario,ÔÇŁ says Dr Meyer.╠ř╠ř
Progress in the field has been slow, as finding alternatives and testing their durability is a lengthy and expensive process. ÔÇťFor instance, creating a new hydrogen fuel cell catalyst can take up to a year, and then even longer to understand exactly whatÔÇÖs happening using expensive equipment that is hard to access,ÔÇŁ says Dr Meyer.
For Prof. Zhao, Dr Meyer and their team, the answer to addressing the existing issues in the field was to develop a method that allows you to understanding why some catalyst materials are not as stable as platinum.╠ř╠ř
ÔÇťUsing three novel methods that we tested in the lab, we can quickly figure out how stable our platinum-free fuel cell is and most importantly understand why. This approach can be easily adopted by scientists in other labs to gain quick and accurate insights into the efficiency of their fuel cells and catalysts,ÔÇŁ says Prof. Zhao.╠ř
Using these techniques, the team revealed that up to 75 per cent of the iron-based active sites (the specific locations where the reactions happen) become inactive in the first 10 hours of running the fuel cell, due to the loss of iron active sites. This is then followed by carbon corrosion becoming the predominant degradation mechanism.╠ř
ÔÇťThis is particularly significant as we can pinpoint exactly what is happening and when it is happening. If we develop a material that has more stable active sites, we should see a slower decay in the first 10 hours, while carbon corrosion may have a similar trend,ÔÇŁ says Dr Meyer.╠ř
ÔÇťBy allowing precise tracking of the degradation mechanisms, we expect that the research field will be able to make new materials targeting these stability issues. As a result, we believe our approach will help improve the stability of platinum-free catalysts and give this field a brighter future.ÔÇŁ╠ř
Read more: ╠ř
While this is a major step in the field of hydrogen fuel cells, Prof. Zhao, Dr Meyer, and their team have their sights set on the next goal.
ÔÇťWe are developing a catalyst where weÔÇÖre combining different metals to increase the stability of the catalysts,ÔÇŁ says Prof. Zhao. "Using the process weÔÇÖve developed here, weÔÇÖre able to get quick, reliable insights into the stability of these low-cost non-platinum catalysts. This gets us some exciting results understanding what is happening.ÔÇŁ╠ř
The team is also focusing on ways they can increase the scalability of the low-cost, platinum-free hydrogen fuel cell catalyst from the lab to a product that could be used to power real devices and, one day, power transport on our roads.╠ř╠ř